Research Focus
Jay D. Keasling is a global leader in synthetic biology and metabolic engineering whose work has transformed how we produce medicines, fuels, and sustainable chemicals. He pioneered the engineering of yeast to produce natural product therapeutics, and his lab continues to advance technologies that convert renewable resources into biofuels, bioproducts, and next-generation therapeutics.
His expertise includes:
- engineering pathways for high-level and large-scale production of hydrocarbons
- using functional genomics to characterize engineered organisms
- developing biological components (gene expression control systems, metabolic pathways, enzymes) and host cells (e.g. Escherichia coli, Saccharomyces cerevisiae, Pseudomonas putida, Streptomyces) for production of biofuels and bioproducts.
Projects
The focus of the work of the Keasling Laboratory in JBEI is to produce advanced biofuels and bioproducts using polyketide synthases (PKSs). PKSs are responsible for synthesis of countless natural products that have found use as human therapeutics (e.g., antibiotics, anticancer, etc.). PKSs and their relatives, the non-ribosomal peptide synthases (NRPSs), have incredible chemical flexibility. What’s more, the sequence of the subunits in the PKS/NRPS can be directly mapped onto the resulting chemical that they produce, and parts of PKSs and NRPSs can be recombined into unnatural enzymes that will produce different (unnatural) chemicals. The Keasling Laboratory in JBEI is recombining PKSs/NRPSs to produce chemicals that might otherwise be produced from petroleum (e.g., hydrocarbon fuels, adipic acid, etc.) as well as chemicals that could never be produced from petroleum using chemistry that is available today. Examples of work in the Keasling Laboratory’s efforts in JBEI include:
- development and optimization of hybrid PKSs to produce a variety of fuels and chemicals,
- development of pathways to precursors that will feed the hybrid PKSs to produce the desired products and
- expression of the engineered PKSs and precursor pathways in JBEI hosts to produce these molecules from lignocellulosic biomass.
Featured Media
Looking at the Future of Synthetic Biology (Video, NHK-World Japan)

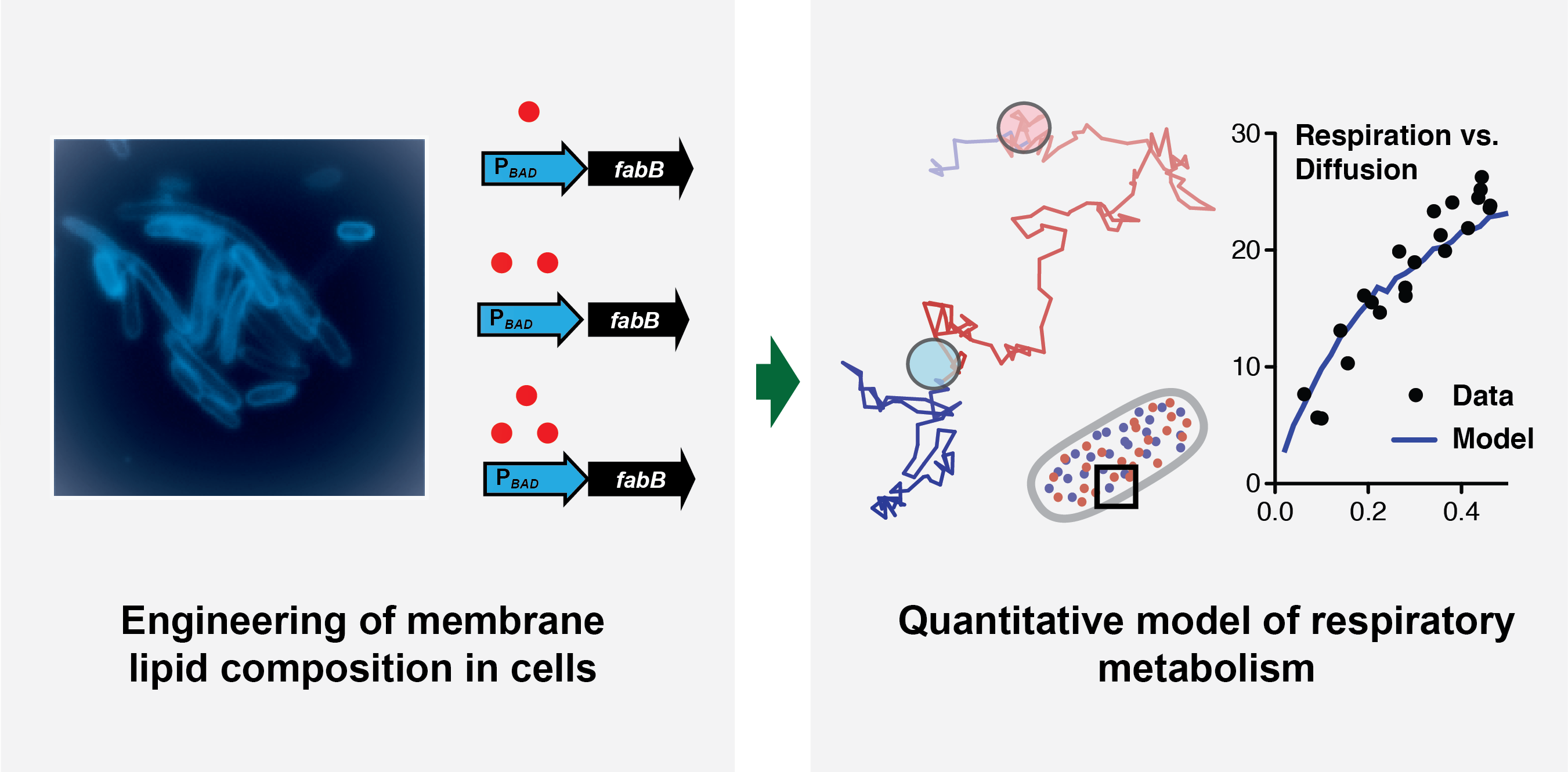
Metabolic Engineering of Lipids Improves the Respiratory Function of Biofuels and Bioproducts Hosts
Faster, Cheaper, Better: A New Way to Synthesize DNA
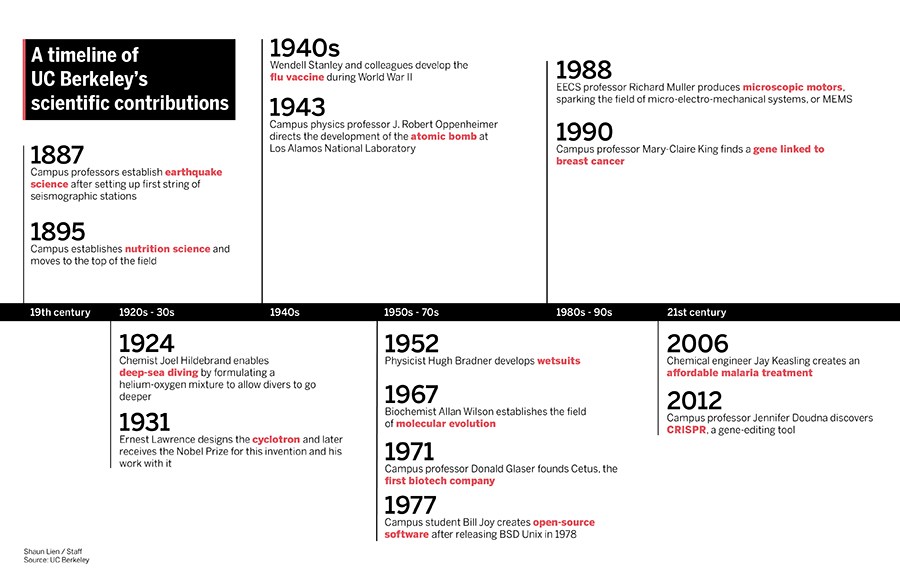
From cyclotrons to wetsuits: A brief history of UC Berkeley’s scientific endeavors
Brewing hoppy beer without the hops
Keasling Wins Israel’s Top Prize in Alternative Fuels
The Economist Recognizes JBEI’s CEO Jay Keasling for Anti-Malarial Effort
Life-Saving Dividends for Synthetic Biology Research: Microbial-Based Antimalarial Drug Shipped to Africa

Keasling Wins 2014 Eni Award’s Renewable Energy Prize
Less Toxic Metabolites, More Chemical Product
Turning Sugar into High Performance Fuel: CNN’s The Next List Profiles Jay Keasling
Launch of Antimalarial Drug a Triumph for Synthetic Biology
Jay Keasling Wins Heinz Award
Featured Publications
- “Engineering controllable alteration of malonyl-CoA levels to enhance polyketide production” Nature Chem. Biol. (2025)
- “Retrobiosynthesis of unnatural lactams via reprogrammed polyketide synthase”, Nature Catalysis (2025)
- “A polyketide-based biosynthetic platform for diols, amino alcohols and hydroxy acids”, Nature Catalysis (2025)
- “Complete integration of carbene-transfer chemistry into biosynthesis”, Nature (2023)
- “Expanding Extender Substrate Selection for Unnatural Polyketide Biosynthesis by Acyltransferase Domain Exchange within a Modular Polyketide Synthase”, J. Am. Chem (2023)
- “Unnatural biosynthesis by an engineered microorganism with heterologously expressed natural enzymes and an artificial metalloenzyme”, Nature Chem. (2021)
- “Short-chain ketone production by engineered polyketide synthases in Streptomyces albus“, Nature Communications (2018)
- “Viscous control of cellular respiration by membrane lipid composition”, Science, (2018)
- “De novo DNA synthesis using polymerase-nucleotide conjugates”, Nature Biotechnology (2018)
- “Industrial brewing yeast engineered for the production of primary flavor determinants in hopped beer”, Nature Communications (2018)
- “Heterologous gene expression of N-terminally truncated variants of LipPKS1 suggest a functionally critical structural motif in the N-terminus of modular polyketide synthase”, ACS Chem. Biol. (2017)
- “Engineering high-level production of fatty alcohols by Saccharomyces cerevisiae from lignocellulosic feedstocks”, Metab. Eng. (2017)
- “Alteration of polyketide stereochemistry from anti to syn by a ketoreductase domain exchange in a Type I modular polyketide synthase subunit”, Biochemistry (2016)
- “Engineering a polyketide synthase for in vitro production of adipic acid”, ACS Synth. Biol. (2016)
- “Engineering dynamic pathway regulation using stress-response promoters”, Nature Biotechnology (2013)
- “Microbial engineering for the production of advanced biofuels”, Nature (2012)
- “Synthesis: A constructive debate”, Nature (2012)
- “Manufacturing molecules through metabolic engineering”, Science (2010)
Featured Intellectual Property
- Biosynthetically Produced Pinene for Jet Fuel or Chemical Applications
- Methods for Increasing Production of 3-methyl-2-Butenol Using Fusion Proteins
- 5-Carbon Alcohols for Drop-in Gasoline Replacement
- Biosynthetically Produced Pinene for Jet Fuel or Chemical Applications
- Rationally Designed Synthetic Transcription Factors
- Producing Dicarboxylic Acids Using Polyketide Synthases
- Alternative Diesel Fuel from Biosynthetic Bisabolene
- Using Polyketide Synthases to Produce Advanced Biofuels
- Efflux Pumps to Increase Microbial Tolerance and Biofuel Production