-By Lida Gifford
New crystallography finding by JBEI and GLBRC benefits bioenergy industry
During the kraft process used to convert wood into wood pulp, the structural material lignin is partially converted into molecules like stilbene. Stilbenes are also naturally occurring in plants and some bacteria, and may play a role in plant pathogen resistance.
Currently, the deconstruction of plant biomass into cellulose and lignin is an expensive process. Lignin accounts for about 30 percent of plant cell wall carbon, and its conversion into chemicals or fuels could have a significant positive impact on the economics of processing lignocellulosic biomass. Enzymes capable of producing useful compounds from the breakdown of stilbenes and similar molecules could be employed for this. Collaborators from two of the Department of Energy Bioenergy Research Centers now have gained first-hand insight into how a stilbene cleaving oxygenase (SCO) carries out this unusual chemical reaction.
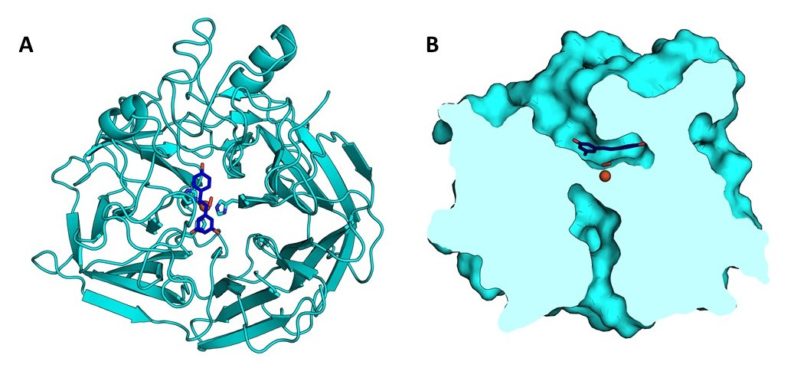
Researchers from the Joint BioEnergy Institute (JBEI) and the Great Lakes Bioenergy Research Center (GLBRC) report the atomic-level structure of NOV1, an SCO that breaks down a stilbene substrate into two smaller compounds. Paul Adams, vice president for technology at JBEI and the senior author of the study, explained, “In order to get a complete picture of how this enzyme works, we solved the structure of NOV1 in complex with a representative stilbene substrate (resveratrol), a representative product (vanillin), as well as in its unbound form.”
“When we studied the structures of NOV1, we saw a ternary complex of protein, oxygen, and either the substrate or product in the active site. This has not been seen previously in any crystal structures of related carotenoid cleavage oxygenases (CCO),” said co-first author Ryan McAndrew, project scientist at JBEI. “Despite the fact that it is similar to CCOs, this NOV1 structure shows several key differences indicative of their substrate preferences and how the enzyme carries out its reaction.”
This enzyme’s active site contains a coordinated iron atom that forms a stable complex when exposed to nitric oxide. This allowed for study by electron paramagnetic resonance (EPR) spectroscopy, which confirmed the configuration of the atoms in the crystal structure active site and provided information useful for elucidating the enzyme mechanism. GLBRC Deconstruction lead Brian Fox said, “Through our work, we were able to propose a mechanism for the reaction that requires dioxygen and the unique arrangement shown in the active site by the crystal structure. This gives new insight into how an SCO might be used to generate desirable bioproducts. As an added benefit, this work helps us understand related enzymes like carotenoid cleavage oxygenases, which produce vitamin A and retinal found in the eye.”
Cost reduction of the plant biomass breakdown and conversion of deconstruction byproducts such as lignin into chemicals are core missions of the Bioenergy Research Centers. The result of this interdisciplinary collaborative study is another step toward finding ways to change a very abundant material like lignin into beneficial valuable bioproducts. “Ultimately, enzymes like NOV1 could produce value in the biological production of molecular fragments derived from lignin,” said Adams. “This would contribute to the sustainable operation of a biorefinery for the production of biofuels and other bioproducts.”
Click here to read more about these recent developments.