A novel approach to synthetic biology could revolutionize how scientists improve plants for bioenergy and agriculture
For the first time, researchers at JBEI have developed a genome-scale way to map the regulatory role of transcription factors, proteins that play a key role in gene expression and determining a plant’s physiological traits. Their work reveals unprecedented insights into gene regulatory networks and identifies a new library of DNA parts that can be used to optimize genetic engineering efforts in plants.

Niklas Hummel (Image courtesy of Niklas Frederik Christopher Hummel)
“Transcription factors regulate things like how plants grow, how much fruit they produce, and what their root architecture looks like,” said Niklas Hummel, lead author of a study on the research in the journal Cell Systems and a research associate at the Department of Energy’s Joint BioEnergy Institute (JBEI), which is managed by Lawrence Berkeley National Lab. “By deciphering their regulatory role, we can identify new strategies to engineer more drought-resilient bioenergy crops and other plants with improved agronomic traits.”
Hummel and study senior author Patrick Shih, a faculty scientist in Berkeley Lab’s Biosciences Area and Director of Plant Biosystems Design at JBEI, set out to develop a method to characterize a large number of transcription factors in a plant simultaneously. While methods to do this exist for other model organisms, such as animals, insects, and fungi, applying them to plants has been challenging, due to their complexity and the disrupting presence of cell walls.
“To date, these kinds of studies have really been done piecemeal in plants, where we only understood the function of a particular transcription factor because one group of researchers has focused on it for many years,” Shih said, who is also an investigator at the Innovative Genomics Institute. “So, what we tried to do instead was come up with a way to map the activity of hundreds of these transcription factors in a plant at the same time.”
To address this challenge, Hummel and Shih employed a transient expression system they had previously developed for building synthetic biology tools in plants. Here, they used the system to characterize, in parallel, a network of over 400 transcriptional effector domains in the tobacco plant Nicotiana benthamiana, a feat never before achieved in plant synthetic biology.
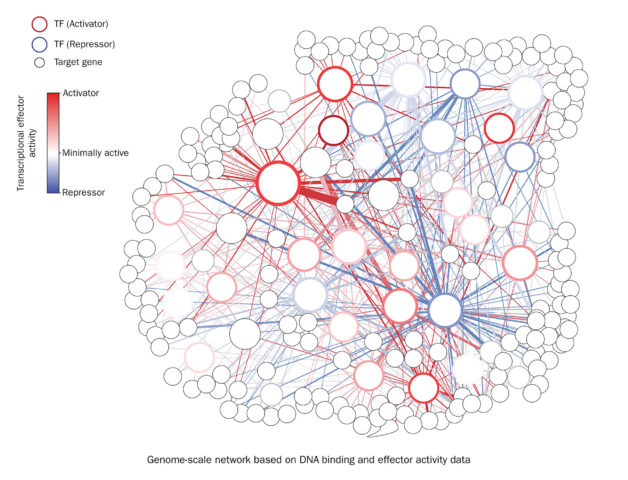
Studying the potential of hundreds of plant transcription factors to turn on and off genes allows scientists to annotate regulatory activity in previously described gene networks. This will help scientists understand how large groups of transcription factors regulate their target genes during drought stress and varying nutrient availability. (Credit: Niklas Frederik Christopher Hummel/Berkeley Lab)
They then went on an extensive literature review to try to match the function of the transcription factors they had identified en masse with previous work done identifying the function of individual transcription factors in their network.
“We were able to show this is what people have seen when they individually studied the role transcription factors play in gene expression, and this is what we have seen when we have studied them in parallel,” Shih said. “It actually ended up aligning really well. This makes us confident that we can integrate our dataset into gene regulatory networks to identify key transcription factors for engineering important plant traits.”
One surprising aspect of the study was the discovery of similar mechanisms of transcriptional regulation across distantly related eukaryotes. By examining the function of transcription factor regulation in both plants and yeast, the researchers found shared functionality, highlighting the presence of deeply conserved mechanisms of gene regulation.
“We were surprised to see that many transcription-factor regulatory domains functioned the same across plants and yeast,” Hummel said. “We then expanded upon this to demonstrate how machine learning algorithms trained on yeast datasets could work to identify regulatory domains in plants.”
The findings of the study have important implications for agriculture and sustainability. Transcription factors play a crucial role in determining important traits in plants, so understanding how they work will help scientists develop strategies to improve agricultural practices and address environmental challenges.
Looking ahead, the researchers aim to expand their approach to study all transcription factors in Arabidopsis, a widely studied model plant species. This will further accelerate understanding of plant-specific gene regulation and facilitate advancements in the field of plant biology.
“Our ability to engineer and modify plants is dependent on our basic understanding of how various traits are regulated,” Shih said. “By understanding how key transcription factors may be master regulators of traits of interest, we could identify new strategies to improve bioenergy relevant traits.”
This work was supported by the Department of Energy’s Office of Science. JBEI is a DOE Bioenergy Research Center.
This article was written
